 Scotch whisky is usually diluted before filled into casks. The usual filling strength for malt whiskies is 63,4-63,5% ABV (111 UK proof) and 68% ABV for grain whiskies. These are industry standards based probably on the history, minimizing evaporation losses and warehousing costs, but also creating an acceptable flavour profiles and uniform products for the blending industry. Bourbon and corn whiskey producers can by law fill at a maximum of 125 US proof (62,5%) and the Irish have commonly filled casks at 71%. Before bottling whisky is usually diluted to 40% or 43%.
Scotch whisky is usually diluted before filled into casks. The usual filling strength for malt whiskies is 63,4-63,5% ABV (111 UK proof) and 68% ABV for grain whiskies. These are industry standards based probably on the history, minimizing evaporation losses and warehousing costs, but also creating an acceptable flavour profiles and uniform products for the blending industry. Bourbon and corn whiskey producers can by law fill at a maximum of 125 US proof (62,5%) and the Irish have commonly filled casks at 71%. Before bottling whisky is usually diluted to 40% or 43%.Known unusual cask filling strengths are Aberlour 69,1% (121 UK proof), Bruichladdich (undiluted 70-72%), Port Charlotte (undiluted, probably over 70%), Glenrothes (63,5% and "some casks" undiluted about 70%) and Glen Scotia 62,5%. Bladnoch has experimented with higher and lower strengths, but apparently is now filling all the casks at 63,5%. Grain spirits are usually filled at 68%, but North British fills at 62-68% and Girvan at 74% (at least the ones going to Grant's). Most malt distilleries use 63,4-63,5% fills.Since 1848 the strength of whisky warehoused in a distillery was legally from 22 under proof (44,6%) to 25 over proof (71,4%) and the maximum strength of whisky sold out of a distillery was 111 proof, so at least the grain distillers were diluting their new make. Irish whisky was said to be warehoused at 14-16 over proof (65,1-66,3% abv) in 1808 and at the same time Scottish whisky for home consumption was taxed at 107 proof (61,1%). Illicit distillers most likely did not dilute their spirits, but the small stills and wide cuts probably did not produce much higher abv.
Ross states in 1970 that Scottish malt whisky is filled at 11-12 over proof (63,4-64% abv) and Irish pot still at 25 over proof (71,4% abv). The dilution to an uniform strength probably eased the common practice of exchanging cask between the different blenders. During the years of Scottish whisky overproduction in the late 70's and early 80's at least DCL "uncommonly" filled casks at full proof to cut cask- and warehousing costs. In the early 60's there were problems acquiring enough casks and that would probably have had the same effect on filling strengths. It is likely that dilution of malt whiskies down to 63,5% has been used at least for the most part of the 20th century. During the WW I the maximum bottling strength was temporarily (1915-16) adjusted to 35 under proof (37,2% abv). The alcoholic strength was indicated usually in Sykes proof until 1980, after that it has been replaced with alcohol per volume (abv).
 |
| Entry proof of whisky and congener extraction (Reazin 1981) |
 |
| Extraction of sugars (Reazin 1981) |
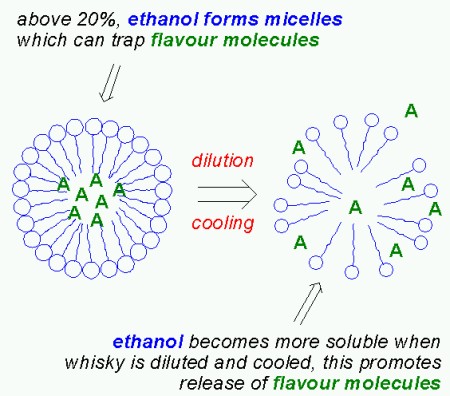 |
| Ethanol micelles (from blog.khymos.org) |
 |
| Effect of ethanol concentration on the maturation of cognac (Cantagrel & Galy 2003) |
 |
| Cutty Sark ad from 1977 |
References and further reading
Conner JM, et al. Release of distillate flavour compounds in Scotch malt whisky. J. Sci. Food Agric., 1999; 79; 1015–1020
Conner JM, et al. Agglomeration of ethyl esters in model spirit solutions and malt whiskies. J. Sci. Food Agric., 1994; 66; 45–53
Conner JM, et al. Interactions between ethyl esters and aroma compounds in model spirit solutions. J Agric Food Chem 1994;42;2231-4
Conner JM, et al. Contributions of distillate components to disperse phase structures in model spirit solutions. J Agric Food Chem 1998;46;1292-6
Conner JM, et al. Agglomeration of ethyl esters in model spirit solutions and malt whiskies. J. Sci. Food Agric., 1994; 66; 45–53
Conner JM, et al. Interactions between ethyl esters and aroma compounds in model spirit solutions. J Agric Food Chem 1994;42;2231-4
Conner JM, et al. Contributions of distillate components to disperse phase structures in model spirit solutions. J Agric Food Chem 1998;46;1292-6
Lea GH, Piggott JR. Fermented beverage production 2nd ed. Kluwer Acad 2003.
Piggott JR, et al. The influence of non-volatile constituents on the extraction of ethyl esters from brandies. J. Sci. Food Agric., 1992; 59: 477–482
Reazin GH. Chemical mechanisms of whiskey maturation. Am J Enol Vitic 1981;32;4;283-9
Ross, J. Whisky. Routledge & Kegan Paul Books 1970
Udo M. The Scottish whisky distilleries. Black&White Publishing 2006.Taylor AJ, Mottram DS. Flavour Science: Recent Developments. Woodhead Publishing 1996.